Sherab Dorji1 , Karma Tenzin2
, Karma Tenzin2
 ,
Somboon Kietinun3, Pratya Phetkate3, Kusuma Sriyakul4
,
Somboon Kietinun3, Pratya Phetkate3, Kusuma Sriyakul4
1Faculty of Traditional Medicine, Khesar Gyalpo University of Medical Sciences of Bhutan, Thimphu, Bhutan
2Faculty of Undergraduate Medicine, Khesar Gyalpo University of Medical Sciences of Bhutan, Thimphu, Bhutan
3Chulabhorn International College of Medicine, Thammasat University, Rangsit, Thailand
Corresponding author: Sherab Dorji, Faculty of Traditional Medicine, Khesar Gyalpo University of Medical Sciences of Bhutan, Thimphu, Bhutan.
Email: sherdho@kgumsb.edu.bt
DOI: https://doi.org/10.47811/bsj.0008050401
ABSTRACT
Introduction: In Bhutanese Traditional Medicine, Chingdug therapy is available for the treatment of chronic low back pain. In this study, the effectiveness of Chingdug was compared with self-administered Diclofenac 1% gel in the management of nonspecific low back pain.
Method: A randomized non-inferiority trial was conducted among patients with nonspecific low back pain at National Traditional Medicine Hospital, Bhutan. Participants were randomly allocated to the Chindgug therapy (n = 30) delivered as per standard protocol and self-administered Diclofenac 1% gel (n = 30) groups. Primary outcomes were at baseline and weekly for two weeks.
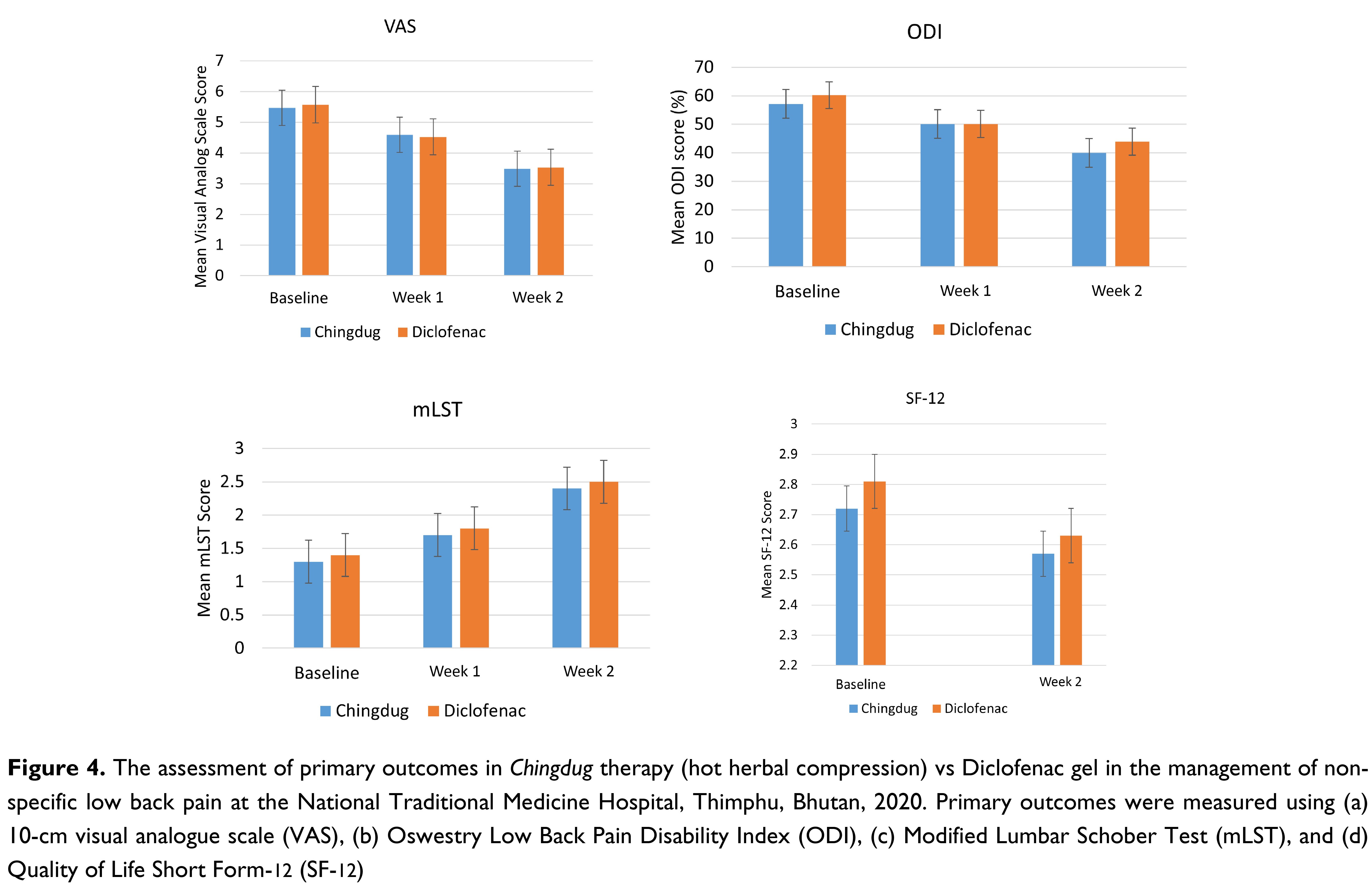
Results: At the end of Week 2, 83.33% in the Chingdug group had a moderate score on Visual Analogue Scale compared to 63.33% in the Diclofenac group (p = 0.080). The mean Visual Analogue Scale score reduced from 5.47 at baseline to 3.49 at Week 2 (p < 0.05) in the Chingdug group while it reduced from 5.67 to 3.63 in the Diclofenac group (p < 0.05). There was a decrease in the Oswestry Disability Index score from 57.17 at baseline to 39.93 at Week 2 in the Chingdug group (p < 0.05) while it reduced from 60.20 to 49.93 in the Diclofenac group (p < 0.05). There was an increase in Modified Lumbar Schober Test score from 1.22 at baseline to 2.29 at Week 2 in the Chingdug group while it increased from 1.23 to 2.33 in the Diclofenac group.
Conclusions: Both Chingdug therapy and diclofenac gel showed a reduction in pain symptoms and disability and an improvement in lumbar range of motion.
Key words: Alternative Medicine; Analgesia; Anti-inflammatory Agents; Chronic Pain; Herbal Therapy; Pain Perception; Thermotherapy
INTRODUCTION
Low Back Pain (LBP) is one of the leading causes of disability globally [1], and ranks as the third leading cause of hospital visits in Bhutan [2]. In Bhutan, the Ministry of Health reported a total of 91,409 patients with musculoskeletal disorders in the country, where LBP was a major part of it [3]. LBP is reported among the top ten diseases treated at the National Traditional Medicine Hospital, with 4615 patients treated for LBP in 2017 and 3518 patients in 2022 [4, 5].
LBP is defined as a discomfort localized to the anatomic area of the lumbosacral region, with or without radiation to the legs [6, 7]. In some patients with LBP, a patho-anatomical relationship can be demonstrated between the pain and one or more pathological processes, including compression of neural structures, joint inflammation, or instability of one or more spinal motion segments [6]. Back pain is classified as nonspecific when there is no clear causal relationship between the symptoms, physical findings, and imaging findings [7].
Guidelines for the treatment of LBP recommend using paracetamol as the first choice, followed by Non-Steroid Anti‐Inflammatory Drugs (NSAIDs) or opioids [6]. The use of NSAIDs is based on the analgesic and anti-inflammatory mechanisms of the drug. NSAIDs are often available as over-the-counter medicines but are also associated with adverse events, such as gastrointestinal and cardiovascular events. Diclofenac 1% gel is one of the over-the-counter lotions available for musculoskeletal pain.
In Bhutanese Traditional Medicine (TM), there are various therapies available to treat LBP: Jukpa (བྱུག་པ། massage), Luejong (ལུས་སྦྱོང་། physical exercise), Chulum (ཆུ་ལུམས། herbal bath), Langlum (རླངས་ ལུམས། steam bath), Langdug (རླངས་དུགས། local steam), Chingdug (བཅིངས་དུགས། Hot Herbal Compression), and Serkhap (གསེར་ཁབ། Gold Needle) [8]. According to a survey among 226 patients with LBP visiting NTMH in 2015, Hot Herbal Compression was the most preferred choice of therapy [9]. However, there is no study to assess the efficacy of Chingdug in the treatment of LBP. This study was conducted to assess the efficacy of Hot Herbal Compression, compared against Diclofenac gel, in the management of non-specific LBP among patients visiting the National Traditional Medicine Hospital, Thimphu, Bhutan.
METHOD
Study Design
This was a randomized non-inferiority trial to evaluate efficacy of Chingdug, hot herbal compression in the treatment of nonspecific LBP in comparison with Diclofenac 1% gel.
Study setting
The research was conducted at National Traditional Medicine Hospital, Thimphu, Bhutan between May and August 2020. The primary mandate of the hospital is to deliver high-quality TM services to patients from all twenty districts of the country. At present, hospital offers a variety of therapeutic services including acupuncture treatment. The facility receives a daily patient load of 150 to 200 in its outpatient department [10].
Study population
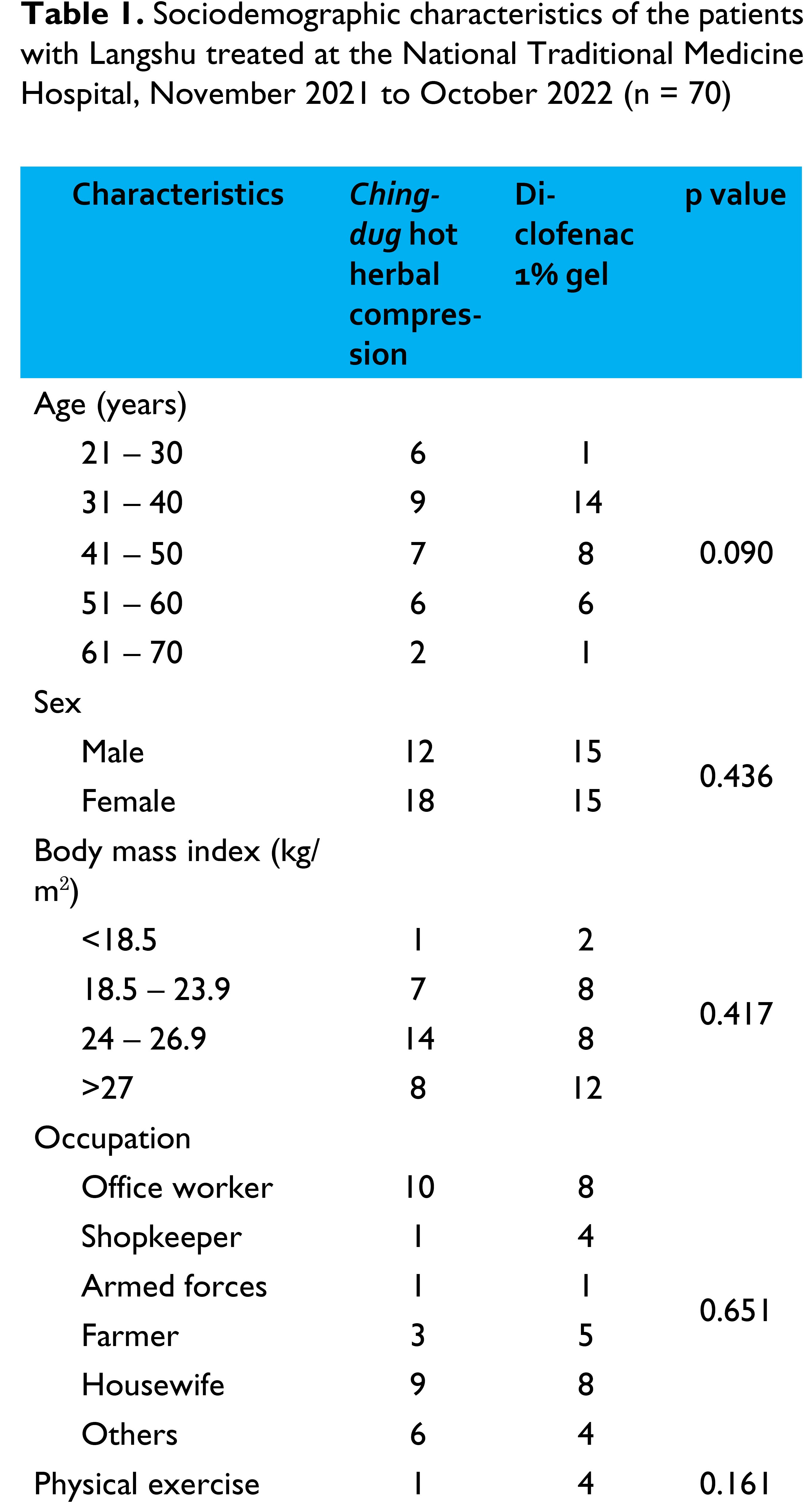
Patients visiting the National Traditional Medicine Hospital with nonspecific LBP during the study period were recruited. A total of 60 participants were enrolled 30 in each group. The inclusion criteria were as follows: both sexes, 20 – 70 years, diagnosed with LBP by TM physicians, pain lasting ≥6 weeks, pain score on Visual Analogue Scale measuring >3 and ≤7 [8]. The exclusion criteria were as follows: injection of local anaesthesia for pain relief within 2 weeks, post-surgical procedures around the spine or in the abdominal area within 3 months, patients with neurological signs, compression of the spinal nerve root, pregnancy or lactation, allergy to herbs used in Chingdug formula and Diclofenac, temperature 100 °F [6, 7], and those receiving any medications or injections.
Sample Size Calculation
The sample size calculated was 60 patients using G*power program with the following assumptions: α = 0.05, effect size = 0.8, power of test (β) = 0.9, and dropout rate = 10%. There were 30 participants were enrolled in each group.
Recruitment and randomization
Participants who fulfilled the inclusion criteria were randomized using a computer-generated simple randomization technique into two groups. Allocation cards were placed in an opaque, sealed and stapled envelopes by a research assistant who wasn’t involved in assessment procedures.
Intervention group: Chingdug
Chingdug is a form of water-based therapy prescribed by Bhutanese TM practitioners. It is a local application of a moist-heated pouch of five prime (or five elixirs) herbs: Ephedra gerardiana Stapf, Juniperus squamata Buch, Myricaria rosea W. W. Smith, Rhododendron anthopogon D. Don and Tanacetum nubigenum DC (Figure 1). In addition to these five major ingredients, 14 minor ingredients are added to pacify different natures of ailments. This product is called dudtsi ngalum (bath of 5-elixirs). Chingdug pack weighs 250 grams of prepared dudtsi ngalum.
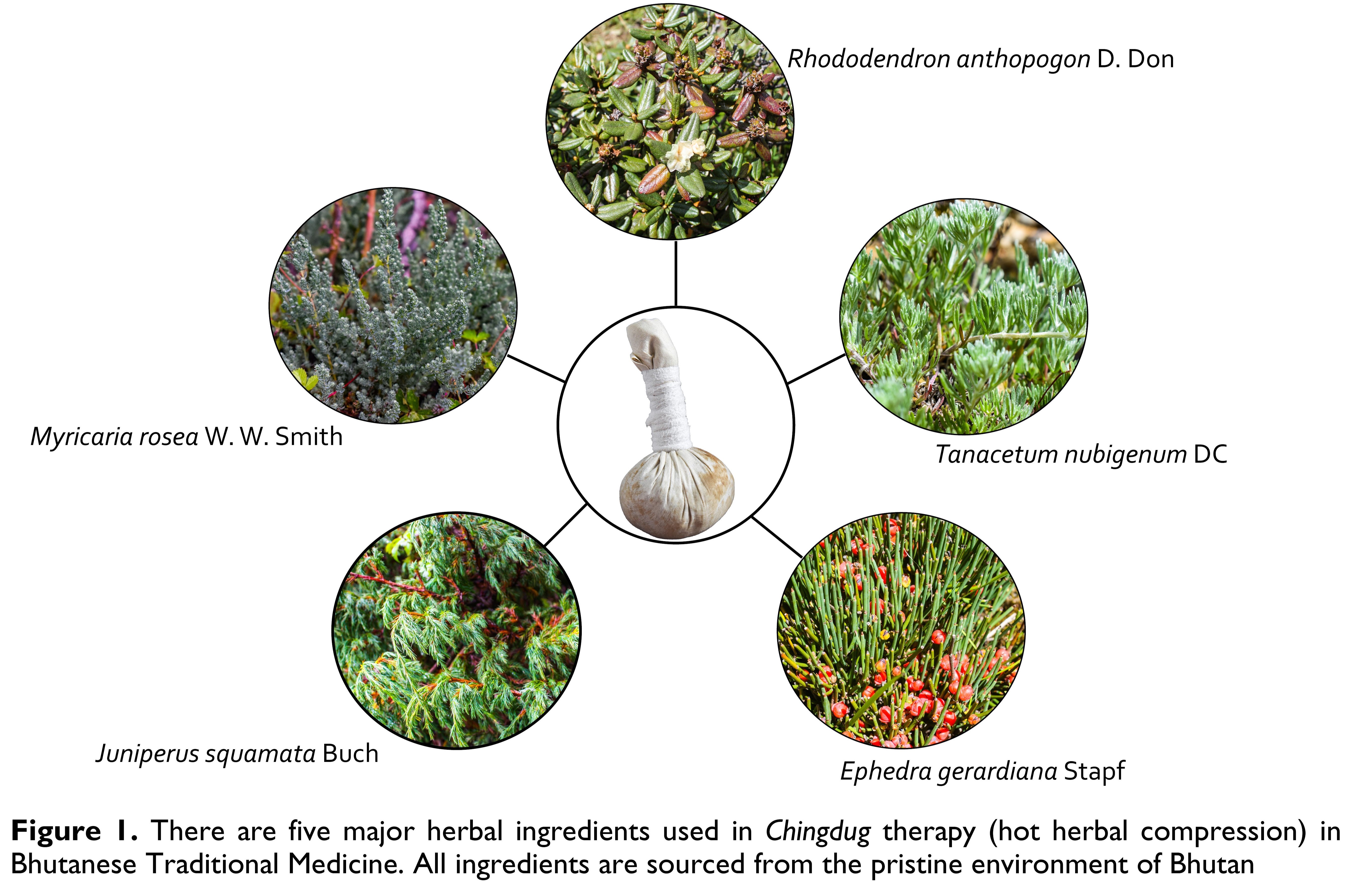
Ephedra gerardiana Stapf is found in Lingshi, Dagala and Bumthang in Bhutan on stony slopes, gravel terraces and in drier areas at altitudes 2400 – 5000 metres above sea level (masl). Its aerial parts possess antibacterial activity against M. leuteus, B. bronchiseptica, S. Setubal at concentrations 5 – 25 mg/mL, anti-inflammatory, anti-influenza and analgesic effects, and anti-metastatic properties [11-13].
Juniperus squamata Buch is found in Lingshi, Dagala and Bumthang in Bhutan in inner valleys and alpine slopes at altitudes 3000 – 4500 masl. Its leaves contain various phytochemicals possessing anti-microbial activities. It is an alternative source of podophyllotoxin and deoxypodophyllotoxin [14].
Myricaria rosea W. W. Smith is found in Lingshi, Haa, Thimphu, Trongsa, Bumthang, Upper Mo Chu, Upper Mangde Chu and Upper Kulong Chu in Bhutan along stream sides at altitudes 3350 – 4250 masl. Its aerial parts are used in the treatment of dugtshad, khrag-tshad and sha-dug and as a febrifuge agent [11].
Rhododendron anthopogon D. Don is found in Haa, Lingshi, Dagala and Phajoding, Punakha, Trongsa, Bumthang, Sakteng and Upper Mo Chu in Bhutan in open hillsides, rocky slopes, cliff ledges at altitudes 3650 – 4700 masl. Dwarf rhododendron scrubs are also found above the tree line. Its flowers are found to possess anti-inflammatory and anti-bacterial properties [11].
Tanacetum nubigenum DC is found in Lingshi, Dagala and Bumthang in Bhutan along stony slopes and sandy grounds at altitudes 3600 – 4800 msal. Its aerial parts are found to possess antiplasmodial, cytoxicity and antimicrobial activities and are used as vulnerary, expectorant, styptic and anti-epistaxis agents [11].
The combination of Chingdug is heated to 46.1 – 52 °C and compressed over the lumbosacral region. Participants received Chingdug as per the Standard Operating Procedure approved by the Department of Traditional Medicine Services, Ministry of Health, Bhutan in 2015 [8]. Chingdug therapy was delivered by TM physicians as follows: Patients were laid on a bed in a prone position, the lumbosacral region was gently cleansed and massaged for 5 minutes, most and heated Chingdug therapy was applied over the lumbosacral region for 15 minutes, and the region was cleaned with a towel. The treatment group received Chingdug compressions of 20 min/session for five days a week i.e. Monday to Friday for two weeks.
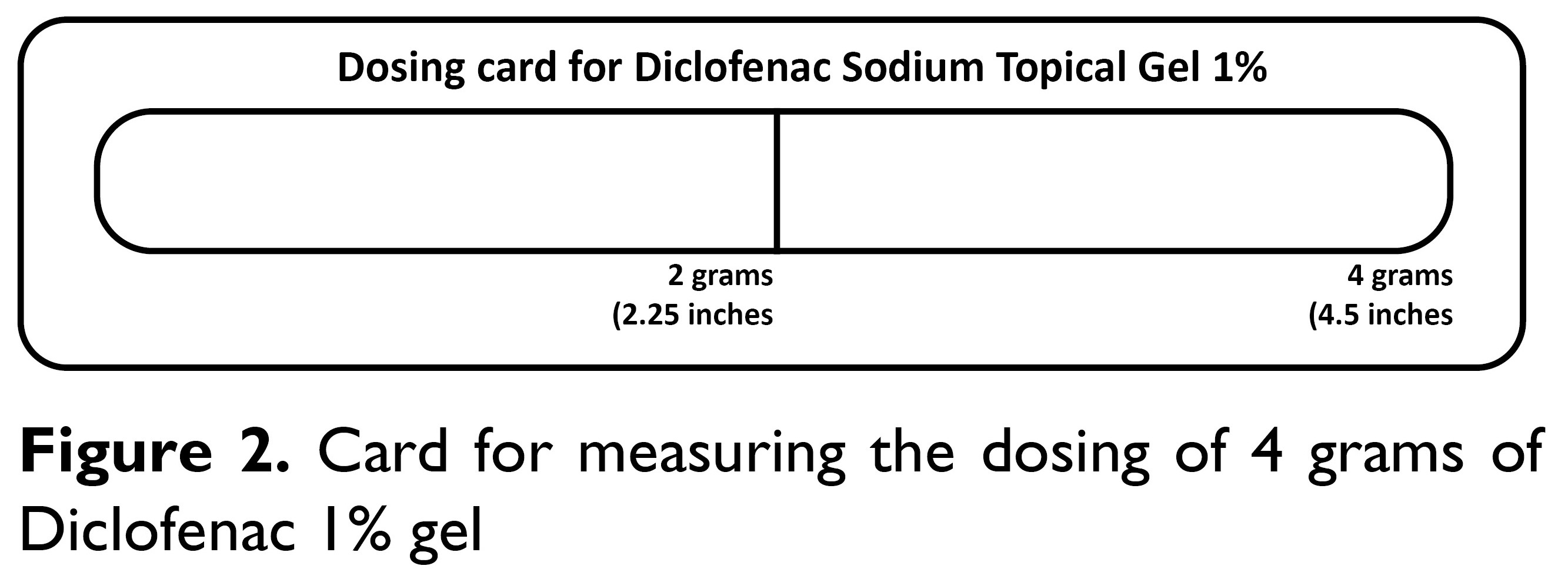
Control group: Diclofenac gelThe control group received Diclofenac 1% gel with an instruction to self-apply on the lumbosacral region, twice a day as recommended by a pharmacist or medical doctor for 2 weeks. The amount of Diclofenac gel was measured by applying it to the oblong area of the dosing card up to the 4-gram line (Figure 2). After having washed hands, the gel from the dosing card was applied to the lumbosacral region area measuring approximately 400 cm2 of skin. To ensure that the participants followed the instructions, participants were briefed and made to sign an agreement consenting to do so.
Primary outcomes were assessed at baseline and weekly for two weeks by trained research assistants. Pain symptoms were assessed using the 10-cm Visual Analogue Scale. This scale is the most frequently used method to assess pain intensity [15]. The patients were instructed to mark a line in each of three different postures to indicate pain severity, and pain was independently evaluated in three different postural situations (in motion, standing, and sitting). It was quantified by measuring the distance in cm from 0 (no pain) to the patient’s marked rating.
Functional disability was assessed using the Oswestry Low Back Pain Disability Index [16]. This is a self-reported questionnaire of a patient’s perceived disability based on 10 areas of pain and daily activities (pain intensity, personal hygiene, lifting, walking, sitting, standing, sleeping, sexual activity, social activity and travelling). Each section is scored on a 6-point scale (0 – 5), with 0 representing no limitation and 5 representing maximal limitation. The subscales combined add up to a total maximal score of 50. The score is then doubled and interpreted as a percentage of the patient-perceived disability (the higher the score, the greater the disability). In cases where patients did not answer all 10 sections, the sum score of the answered sections was divided by the number of completed sections.
The range of motion of lumbar flexion was determined using the Modified Lumbar Schober Test [17]. Patients were asked to stand with their back towards the assessor. The assessor marked a horizontal line at dimples of Venus approximately at the level of L5 as the point of the lumbosacral junction. Point A, 5 cm below the line at dimples of Venus is marked and point B, 10 cm above the line at dimples of Venus is marked. The patient was then asked to touch his/her fingers to toes. By doing so, the distance between the two points (above and below the line of dimples of Venus) increases.
Additionally, quality of life was assessed using the Quality of Life SF-12 questionnaire at baseline and at the end of follow-up [18].
Statistical analysisData were entered into and analysed using SPSS (licensed). Continuous variables are summarized as mean and standard deviation, and categorical variables are summarized as frequency and percentages. In a within-group analysis, the mean values of the Visual Analogue Scale, Oswestry Disability Index and Modified Lumbar Schober Test between baseline and the consecutive weeks were compared by a one-way repeated measures analysis of variance. The comparison between groups was done using t-tests and comparisons within groups using paired t-tests and chi-square tests. P values less than 0.05 were considered statistically significant.
Ethics considerationsThis study was conducted respecting the principles of the Declaration of Helsinki. Ethics approval was obtained from the Research Ethics Board for Health (Ref. No: PO/2019/109, dated 31 January 2020). Administrative approval was obtained from the Policy and Planning Division, Ministry of Health, Royal Government of Bhutan and Medical Superintendent of National Traditional Medicine Hospital, Thimphu, Bhutan. Informed written consent was obtained from the participants as per the consent process approved by the ethics board.
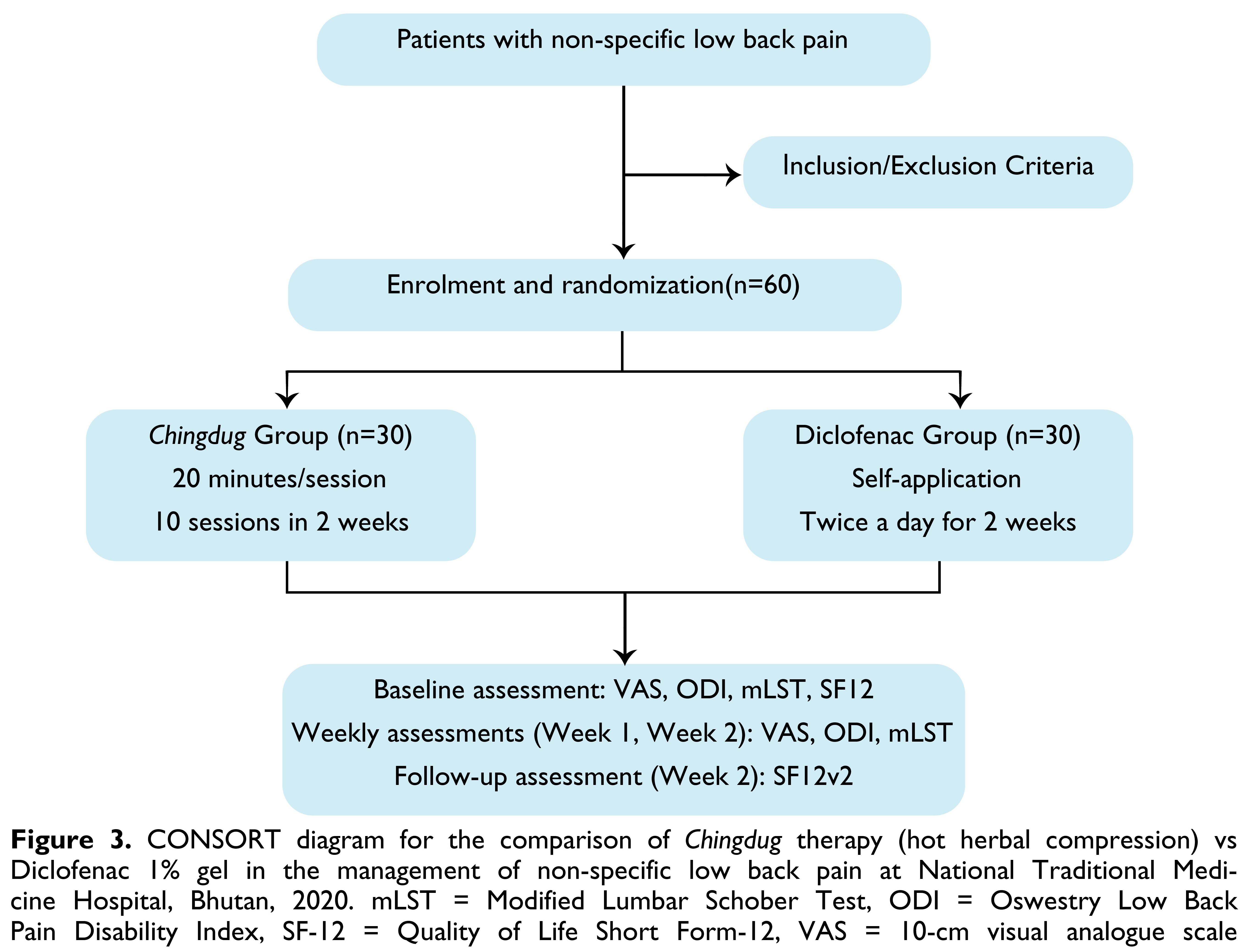
RESULTSThere were 30 patients who completed the follow up sessions in both arms as shown in the CONSORT diagram in Figure 3. The basic characteristics of the groups are shown in Table 1.
At baseline and Week 1, all patients in both groups scored moderate pain on VAS. At the end of Week 2, there were 25 patients (83.33%) who scored better with moderate pain on VAS in the Chingdug group while there were 19 patients (63.33%) who scored moderate pain in the Diclofenac group, p = 0.080.
On the Visual Analogue Scale assessment, Chingdug reduced the pain score from 5.47 ± 0.69 at the baseline to 4.59 ± 0.68 at Week 1, and to 3.49 ± 0.52 at Week 2 (p < 0.05). In the Diclofenac group, the pain score decreased from 5.67 ± 0.59 to 4.62 ± 0.77 at Week 1 and to 3.63 ± 0.63 at Week 2 (p < 0.05) as shown in Table 2. Based on the Oswestry Low Back Pain Disability Index, Chingdug led to a reduction in functional disability score from 57.17 ± 14.87 at baseline to 50.13 ± 14.08 at Week 1 (p < 0.05) and 39.93 ± 11.89 at Week 2 (p < 0.05). In the Diclofenac group, the disability score reduced from 60.20 ± 10.38 at baseline to 50.07 ± 9.73 at Week 1 and 43.93 ± 7.97 at Week 2 (p < 0.05) as shown in Table 2.
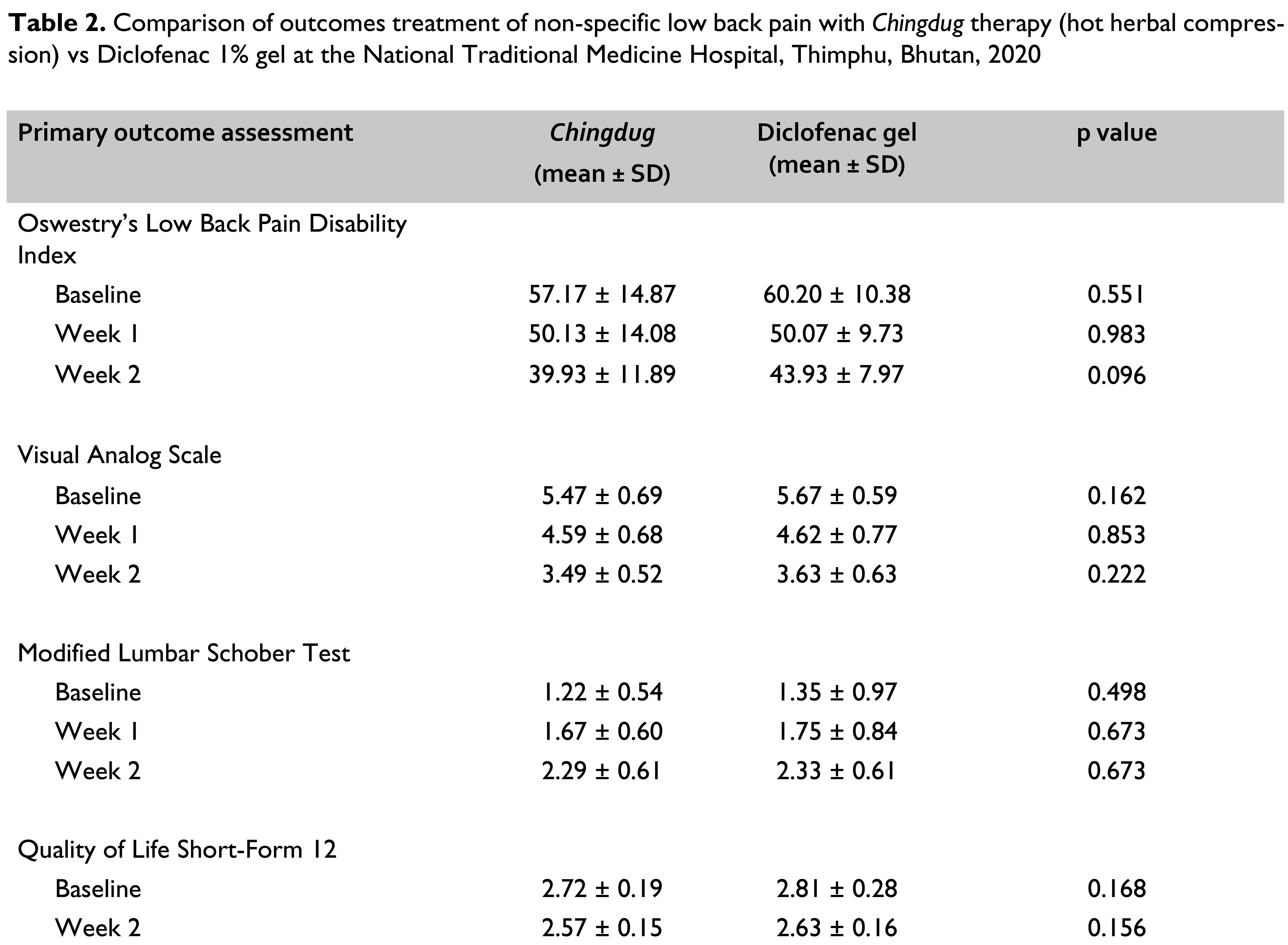
Based on the Modified Lumbar Schober Test, in those receiving Chingdug, there was an increase in the range of motion score from 1.22 ± 0.54 at baseline to 1.67 ± 0.60 in Week 1, which was a 36.99% increment (p < 0.05). There was a further increase in the range of motion by 88.22% at Week 2 with a score of 2.29 ± 0.61 (p < 0.05). In the Diclofenac group, the range of motion at Week 1 had increased by 29.79% (p < 0.05) from 1.35 ± 0.97 at baseline to 1.75 ± 0.84, which was an increment by 29.79%. At Week 2, the range of motion increased by 72.44% to 2.33 ± 0.61. When the two treatment arms were compared, those who received Chingdug had a higher overall increment in the range of motion at follow-up (Table 2).
There was a 5.61% reduction in the SF-12 score in the Chingdug group compared to a 6.55% reduction in the Diclofenac group (Table 2).
DISCUSSIONIn this study, participants in both groups experienced a statistically significant reduction in pain, functional disability, and an increase in lumbar range of motion at follow-up. Based on the findings, Chingdug compression is an effective option for alleviating pain associated with non-specific low back pain. However, there were no statistical differences in the primary outcome assessment of the two groups.
Though the ingredients in Chingdug differ from hot herbal compressions used in other countries, there are similarities noted in the procedure. A randomized control trial done in Thailand assigned 90 participants to 3 groups: a hot compression group, a hot herbal compression group, and a Diclofenac group for the treatment of pain among patients with myofascial pain syndrome [19]. This study reported that both the hot compression and hot herbal compressions were significantly effective in alleviating pain as Diclofenac gel. Similarly, a meta-analysis of clinical effects of Thai herbal compression covering 13 studies found that all the studies analyzed indicated that Thai herbal compression treatments were effective in alleviating pain among patients with osteoarthritis [20].
In our study, Chingdug treatment exhibited the capacity to alleviate non-specific low back pain but was not statistically different from that of the Diclofenac group. Although a definite reason for its effectiveness in the reduction of pain symptoms is yet to be studied, it can be hypothesized that the combination of heat and herbs including Ephedra gerardiana, Juniperus squamata, Myricaria rosea, M. germanica, Rhododendron anthopogon and, Tanacetum nubigenum contributes to resolution of symptoms. Among these five principal ingredients, Ephedra gerardiana is reported to possess anti-inflammatory properties [12, 13]. Therefore, the common indications for Chingdug in Bhutanese TM include post-traumatic pain, swelling of limbs, neurological disorders, muscular dystrophy, trembling, obstinate skin diseases, piles, gouts and arthritis.
The strengths of this study includes the adoption of a standardized protocol for the delivery of Chingdug therapy. While the constituents of are manufactured as per standard national formulary, this is the first time it has been compared to over-the-counter Diclofenac gel in Bhutan. This is of particular importance because the data on the use of over-the-counter Diclofenac is not well documented. Given the improvements in pain score and functional status shown in this study, Chingdug therapy should be made accessible to patients with non-specific low back pain. In addition to improving symptoms, this therapy has the potential to improve the overall efficiency of the health system by reducing the patient load at allopathic medicine hospitals.
In addition to its use in nonspecific low back pain, the use of Chingdug therapy may be explored in chronic pain conditions such as myofascial pain, arthritis and in degenerative conditions. Given the properties contained in the various elements contained in Chingdug and the use of heat therapy, this might also be useful in inflammatory and degenerative conditions resulting from repetitive use injuries or sports injuries. It is, therefore, recommended to evaluate the effectiveness of Chingdug in other similar clinical conditions to reach its benefits to more patients.
As with other Bhutanese TM herbal formularies, there are multiple ingredients included in the Chingdug compressions. All these ingredients are collected from the natural environment based on standard procedures of collection and processing. The use of a combination of ingredients is to re-establish balances in elements and energies to relieve physical ailments. However, potential drug discovery of analgesic and anti-inflammatory agents contained in Chingdug may be possible with further detailed research. In addition, it is also important to study patient preferences given that Diclofenac is easily available over the counter and is self-administered.
This was the first study on the comparison of Chingdug therapy with Diclofenac in Bhutan and only a relatively small sample size could be recruited. In this study, only one standard procedure was employed for the delivery of hot compressions while a combination of variations of the therapy may be recommended. In addition, the outcome assessor could not be blinded and might have contributed to differential subjectivity in some the measures.
CONCLUSION
Chingdug therapy when compared to Diclofenac gel showed reduction of pain and disability and improvements in lumbar range of motion among patients with nonspecific low back pain. This study adopted a standardized protocol for the delivery of Chingdug therapy. It is recommended that Chingdug should be accessible to more patients with nonspecific low back pain. The use of variations of Chingdug therapy and its effectiveness in other chronic pain conditions are recommended.
Acknowledgement
We are grateful to the Faculty of Traditional Medicine, Khesar Gyalpo University of Medical Sciences of Bhutan, National Traditional Medicine Hospital, Bhutan and Chulabhorn International College of Medicine, Thammasat University, Thailand for the support in conducting this study. We also express our gratitude to all patients who participated in this study.
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Research Ethics Board for Health (Ref. No: PO/2019/109, dated 31 January 2020). Administrative approval was obtained from the Policy and Planning Division, Ministry of Health, Royal Government of Bhutan and Medical Superintendent of National Traditional Medicine Hospital, Thimphu, Bhutan. Informed written consent was obtained from the participants as per the consent process approved by the ethics board.
Consent for publication
Not applicable
Competing interests
SD and KT are editors of this journal. SD and KT were blinded from the peer review process of this article.
Funding
There was no funding for this article.
Availability of data materials
The data set is available from the corresponding author upon request.
Author contributions
Conceptualization, data curation, formal analysis, investigation, methodology project administration, resources, software, supervision, validation, visualization, writing – original draft, writing – review and editing: SD
Conceptualization, investigation, methodology, resources, supervision, writing – original draft, writing – review and editing: KT, SK, PP, KS
Received: 01 March, 2024 Accepted: 9 May, 2024 Published online: 16 May, 2024
References
- Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet (London, England). 2018;391: 2368–2383. doi:10.1016/S0140-6736(18)30489-6
- Ministry of Health. Annual Health Bulletin 2018. Thimphu: Ministry of Health, Royal Government of Bhutan; 2018.
- Ministry of Health. Annual Health Bulletin 2018. Thimphu: Ministry of Health, Royal Government of Bhutan; 2019.
- Department of Traditional Medicine Services. Traditional Medicine Patient Report 2017. Thimphu: Ministry of Health, Royal Government of Bhutan; 2017.
- Department of Traditional Medicine Services. Traditional Medicine Patient Report 2022. Thimphu: Ministry of Health, Royal Government of Bhutan; 2022.
- Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet (London, England). 2021;398: 78–92. doi:10.1016/ S0140-6736(21)00733-9
- Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet (London, England). 2017;389: 736–747. doi:10.1016/S0140- 6736(16)30970-9
- Department of Traditional Medicine Services. Standard Operating Procedures for Traditional Medicine Services. Thimphu: Department of Traditional Medicine Services, Ministry of Health, Bhutan; 2015.
- Wangdi S. An Enquiry into the efficacy of Dutsi-nga-lum, a unique healing therapy in the Traditional Bhutanese Medicine. Men-jong So-rig J. 2015;7.
- Ministry of Health. Annual Health Bulletin 2023. Thimphu: Ministry of Health, Royal Government of Bhutan; 2024.
- Wangchuk P. High altitude medicinal plants of Bhutan: An illustrated guide for practical use. Thimphu: Institute of Traditional Medical Services, Royal Government of Bhutan; 2009.
- Hyuga S, Hyuga M, Oshima N, Maruyama T, Kamakura H, Yamashita T, et al. Ephedrine alkaloids-free Ephedra Herb extract: a safer alternative to ephedra with comparable analgesic, anticancer, and anti-influenza activities. J Nat Med. 2016;70: 571–583. doi:10.1007/s11418-016-0979-z
- Hyuga S, Hyuga M, Yoshimura M, Amakura Y, Goda Y, Hanawa T. Herbacetin, a constituent of ephedrae herba, suppresses the HGF-induced motility of human breast cancer MDA-MB-231 cells by inhibiting c-Met and Akt phosphorylation. Planta Med. 2013;79: 1525–1530. doi:10.1055/s-0033-1350899
- Och M, Och A, Cieśla Ł, Kubrak T, Pecio Ł, Stochmal A, et al. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm Biol. 2015;53: 831–837. doi:10.3109/13880209.2014.943246
- Shafshak TS, Elnemr R. The Visual Analogue Scale Versus Numerical Rating Scale in Measuring Pain Severity and Predicting Disability in Low Back Pain. J Clin Rheumatol Pract reports Rheum Musculoskelet Dis. 2021;27: 282–285. doi:10.1097/RHU.0000000000001320
- Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25. Available: https://journals.lww.com/spinejournal/ fulltext/2000/11150/the_oswestry_disability_index.17.aspx
- Tousignant M, Poulin L, Marchand S, Viau A, Place C. The Modified- Modified Schober Test for range of motion assessment of lumbar flexion in patients with low back pain: a study of criterion validity, intraand inter-rater reliability and minimum metrically detectable change. Disabil Rehabil. 2005;27: 553–559. doi:10.1080/09638280400018411
- Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, et al. Reliability, validity, and responsiveness of the short form 12- item survey (SF-12) in patients with back pain. Spine (Phila Pa 1976). 2003;28: 1739–1745. doi:10.1097/01.BRS.0000083169.58671.96
- Boonruab J, Damjuti W, Niempoog S, Pattaraarchachai J. Effectiveness of hot herbal compress versus topical diclofenac in treating patients with myofascial pain syndrome. J Tradit Complement Med. 2019;9: 163–167. doi:10.1016/j.jtcme.2018.05.004
- Dhippayom T, Kongkaew C, Chaiyakunapruk N, Dilokthornsakul P, Sruamsiri R, Saokaew S, et al. Clinical effects of thai herbal compress: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015: 942378. doi:10.1155/2015/942378