J. M. Dahanayake1 , P. K. Perera1
, P. K. Perera1 , P. Galappaththy2
, P. Galappaththy2 , L. D. A. M. Arawwawala3
, L. D. A. M. Arawwawala3
1Faculty of Indigenous Medicine, University of Colombo, Colombo, Sri Lanka
2Faculty of Medicine, University of Colombo, Colombo, Sri Lanka
3Herbal Technology Section, Industrial Technology Institute, Colombo, Sri Lanka
Corresponding author: J. M. Dahanayake, Department of Ayurveda Pharmacology, Pharmaceutics and Community Medicine, Faculty of Indigenous Medicine, University of Colombo, Sri Lanka.
Email: jeevanimd@fim.cmb.ac.lk
DOI: https://doi.org/10.47811/bsj.0027071204
Copyright © 2026 Bhutan Sorig Journal published by the Faculty of Traditional Medicine, Khesar Gyalpo University of Medical Sciences of Bhutan.
This is an open access article under the CC BY-NC-ND license.
ABSTRACT
Introduction: Phyllanthus niruri Linn. (Phyllanthaceae) is widely used in traditional medicine to treat liver, urinary and respiratory conditions. Despite its therapeutic significance, standardized data on its identity and quality parameters remain limited. This study describes its pharmacognostical, physico-chemical, and phytochemical standards of the whole plant used in herbal preparations.
Methods: The plants were collected in whole, authenticated, and subjected to standard pharmacognostical evaluations according to the World Health Organization guidelines and Ayurvedic Pharmacopoeia of India. Qualitative phytochemical screening, heavy metal analysis, Thin Layer Chromatography (TLC) and High-Performance Thin Layer Chromatography (HPTLC) fingerprints were developed for chemical profiling.
Results: The plant exhibited distinct morphological and anatomical characteristics confirming its identity. Physico-chemical tests revealed acceptable total ash (5.26 ±0.10%), water-soluble ash (0.05 ±0.00%), acid-insoluble ash (5.25 ±0.04%) and moisture (11.4 ±0.08%) levels. Hot water (23.02 ±0.80%) and methanol extractive values (10.4 ±0.80%) were higher than cold water (8.90 ±0.10%) and methanol extractive values (7.20 ±1.50%). Phytochemical screening confirmed the presence of phenols, tannins, terpenoids, steroids, and cardiac glycosides. No heavy metals (Lead, Cadmium, Arsenic, Mercury) were detected. TLC profile showed prominent Rf values of 0.30, 0.34 and 0.56 (at 254 nm). Additional prominent spots were observed with Rf values of 0.20, 0.40, 0.50 and 0.80 (at 366 nm) and HPTLC profile showed 10 peaks related to TLC.
Conclusion: Establishing comprehensive pharmacognostical, physico-chemical, phytochemical and TLC-HPTLC fingerprint profiles help authenticate P. niruri and identify genuine plant materials, which is essential for quality assurance in herbal drug production.
Key words: Chromatography; Health Care Quality Assurance; Herbal Medicine; Pharmacognosy; Plant Extracts; Traditional Medicine
INTRODUCTION
The Family Phyllanthaceae comprises approximately 50 – 60 genera and around 2000 species of herbs, shrubs, climbers and trees [1]. The largest genus within the family is Phyllanthus, which alone includes more than 1,000 species, representing a major proportion of family’s diversity [2]. Members of this family are distributed across a wide range of habitats worldwide, particularly in tropical and subtropical regions [3]. The plants of the family Phyllanthaceae are often characterized by the presence of milky latex and unisexual flowers [4]. Phyllanthus niruri Linn. and then member of this Phyllanthaceae family, is commonly known as Pitawakka in the Sri Lankan local language.
This plant is widely used in both Ayurveda and the Sri Lankan traditional system of medicine to treat diseases of the respiratory system, urinary system and liver diseases [5]. P. niruri contains a wide variety of phytoconstituents such as flavonoids, alkaloids, terpenoids, lignans, polyphenols, tannins, coumarins, and saponins, which are responsible for its diversemedicinal properties [6]. P. niruri has been reported to possess hepatoprotective, antiviral, antibacterial, hypolipidaemic, hypoglycaemic, analgesic, anti-inflammatory, cardioprotective, anti-urolithiatic and antihyperuricaemic properties due to its bioactive compounds [7,8].
P. niruri (whole dried plant) is widely utilized in various traditional herbal preparations, including decoctions, powders, pastes, and pills. The fresh plant is used in several external formulations. Correct identification of the plant materials is an important step in the preparation of herbal medicines. At present, adulteration of plant raw materials with low-quality plant materials is a significant problem in the herbal drug industry, which has major impacts on the commercial use of herbal medicines [9]. This issue is further worsened by the misidentification of herbal materials due to a lack of proper knowledge about medicinal plants. Therefore, an examination to determine macroscopic and microscopic characteristics is the initial step for confirming the identity and the degree of purity of herbal materials [10].
Quality assurance of raw materials and herbal medicines is necessary for producing high-quality and effective drugs for treatment. Standardization and quality control serve as essential tools to ensure the consistency, safety, and efficacy of these products. In the background of growing demand for plant-based raw medicines, there is a need to maintain quality standards to ensure consumer safety and therapeutic effectiveness. Therefore, the primary objective of this study was to analyse the physicochemical, phytochemical, and pharmacognostical characteristics of P. niruri to identify its specific diagnostic features and develop standard parameters for the crude drug.These findings aim to support the authentication and quality control of P. niruri in herbal drug manufacturingsystems.
MATERIALS AND METHODS
Collection of plant materials
Whole plants of Phyllanthus niruri Linn. were collected from Herbal Garden of Faculty of Indigenous Medicine, Rajagiriya in Colombo district, Western Province, Sri Lanka (6° 55’ 54.98” N × 79° 50’ 52.01” E) during the period of June – July 2018 and authenticated by the Curator of National Herbarium of Peradeniya (Voucher specimen No. NH/BOT/4/2019-5), Sri Lanka..
Pharmacognostical study of raw material
Plant parts were macroscopically and microscopically identified based on the standard methods mentioned in Ayurvedic Pharmacopoeia of India [11]. All the images presented were taken by the author using a Samsung digital camera (PL 211 Samsung, London, UK).
Physico-chemical studies
Physico-chemical studies of the shade-dried plant parts were done according to the methods mentioned in Ayurvedic Pharmacopoeia of India under herbal drug standardisation guidelines [11]. Detection of foreign matters, hot- and cold-water extractable matter, hot- and cold- methanol extractable matter, total ash, acid-insoluble ash and water-soluble ash, and moisture content were determined.
Detection of foreign matters
A sample of 50 g (W1) of P. niruri Linn. whole plant was spread in a thin layer on a large wooden tray. Then, the foreign matters in the sample were sorted into groups by visual inspection, using a magnifying lens. The sorted foreign matter was weighed (W2), and the percentage of foreign matter was calculated as follows:
Foreign magtter (%)=Determination of Ash Values
Total ash: An empty silica crucible was initially ignited in a muffle furnace at 550 °C for 30 minutes. The ignited silica crucible was then placed inside the desiccator for 30 minutes and weighed. Subsequently, 4 g of the whole powdered plant of P. niruri Linn. was evenly spread in the crucible, kept in muffle furnace for ignition at 550 °C for 4 hours until the ash became totally white, indicating the absence of carbon. After ignition, the crucible was cooled in the desiccator for 30 minutes and immediately weighed to determine the ash content as follows:
Total ash value =Acid-insoluble ash: To the crucible containing the residue after the determination of total ash, 25 mL of 2M HCl was added and boiled gently for 5 minutes using a Bunsen burner. The solution was then filtered using a Whatman Filter Paper No. 42, and the insoluble matter was collected onto it. The insoluble matter retained on the filter paper was washed with hot water, and then the filter paper was transferred to the same silica crucible. This was ignited to a constant weight in the muffle furnace at a temperature not exceeding 450 °C. After ignition, the crucible was cooled in the desiccator for 30 minutes and its weight was measured immediately. The acid-insoluble ash content was calculated as follows:
Acid-insoluble ash =Water-soluble ash:The above procedure was repeated with 25 mL of distilled water to determine the water-soluble ash value as per the method mentioned in API.
Extractable matters
Cold water-soluble extractive value: From the airdried powdered plant material, 4 g was transferred to a conical flask containing 100 mL of distilled water. The flask was shaken frequently for 6 hours, and then it was allowed to stand for 18 hours. The mixture was filtered through a Whatman Filter Paper No. 1, without losing any solvent. A 25 mL aliquot of the filtered solution was transferred to a porcelain dish in which the weight was previously measured. This dish was placed on a water bath, and the solvent evaporated completely. Following this, the dish was dried in the hot air oven at 105 °C for 6 hours and cooled in a desiccator for 30 minutes. The final weight of the dish with the dried extract was recorded, and the cold water-soluble extractive value was calculated.
Cold methanol-soluble extractive value: The above procedure was repeated with 100 mL methanol to find the cold methanol-soluble extractive value.
Hot water-soluble extractive value: From the airdried airdried powdered plant material, 4 g was transferred to a conical flask containing 100 mL of distilled water and obtained the total weight, including the flask. A reflux condenser was attached to the flask and gently boiled for 2 hours. It was allowed to cool and weighed. A 25 mL aliquot of the filtered solution was transferred to a porcelain dish in which the weight was previously measured. This dish was placed in a water bath, and the solvent evaporated completely. Following this, the dish was dried in the hot air oven at 105 °C for 6 hours and cooled in a desiccator for 30 minutes. The final weight of the dish with the dried extract was recorded, and the hot water-soluble extractive value was calculated.
Hot methanol-soluble extractive value: The above procedure was repeated with 100 mL of methanol to find the hot methanol-soluble extractive value.
Preparation of extractsThe air-dried powdered material (200 g) was refluxed with methanol and water for six hours to obtain hot methanol extracts and hot water extracts. Prepared extracts were filtered separately and dried by evaporation using a rotary evaporator (Buchi, Rotavapor R-210, Switzerland), and the obtained extracts were stored in the refrigerator for further investigation.
Qualitative phytochemical analysisQualitative phytochemical analysis of hot methanol and hot water extracts of P. niruri whole plant were performed as per the methods described by Goveas[12] and Dahanayake et al [13] with some modifications to detect the presence of saponins, alkaloids, tannins, phenols, flavonoids, terpenoids, steroids and cardiac glycosides.
Test for Saponins
Frothing test: 2 mL of extract and 5 mL of water were added to a test tube, shaken vigorously and kept for 10 minutes. Persistence of froth for at least 10 minutes indicated the presence of saponins. The froth was mixed with 3 drops of olive oil and shaken vigorously. The formation of emulsion indicated the presence of saponins in the extract.
Test for Tannins
Ferric chloride test: Five drops of FeCl3 were added to the 2 mL of extract and mixed well. Appearance of a blue-black precipitate indicated the presence of tannins.
Lead acetate test:
Three drops of Pb(OAc)2 were added to 5 mL of extract and mixed well. Formation of a yellow or fluorescent yellow precipitate indicated the presence of tannins.Vanillin test:
A few drops of 10% vanillin in ethyl alcohol and concentrated HCl were added to 2 mL of extract and mixed well. The appearance of red colour indicated the presence of tannins. Test for AlkaloidsMayer’s reagent test:
Six drops of Mayer’s reagent and 1% HCl were added to 2 mL of extract and mixed well. Cream-pale yellow precipitate indicated the presence of alkaloids.Picric acid test:
A few drops of picric acid were added to 2 mL of extract and mixed well. Formation of a yellow crystalline precipitate indicated the presence of alkaloids.Tannic acid test:
A few drops of tannic acid were added to 2 mL of extract and mixed well. Formation of a yellow crystalline precipitate indicated the presence of alkaloids.Wagner test:
Two drops of Wagner reagent were added to 2 mL of extract and mixed well. Appearance of a dark red colour indicated the presence of alkaloids. Test for FlavonoidsAmmonia test:
5 mL of dilute ammonia solution was added to 3 mL of extract followed by the addition of conc. H2SO4. The disappearance of yellow colour on standing indicated the presence of flavonoids.Aluminium Chloride Test:
A few drops of 1 % Aluminium solution were added to 2 mL of extract and mixed well. Yellow colour indicated the presence of flavonoids.Shinoda Test:
5 mL of extract was added to a test tube containing a piece of metallic Mg and three drops of conc. HCl, and heated. The extract produced a red-orange colouration in the Shinoda test, indicating the presence of flavonoids. Test for PhenolsFolin reagent test:
A few drops of Folin reagent were added to 2 mL of extract and mixed well. Blue colour indicated the presence of Phenolics.Pb(OAc)2 test
Three drops of Pb(OAc)2 were added to 2 mL of extract and mixed well. Formation of a yellow precipitate indicated the presence of phenols.Vanillin test:
A few drops of 10% vanillin in ethyl alcohol and conc. HCl were added to 2 mL of extract. The appearance of pink colour indicated the presence of phenolic acid. Red-pink colour indicated the presence of phenyl propene..FeCl3 test:
Five drops of FeCl3 were added into 2 mL of extract and mixed well. Appearance of a green or blue colour indicated the presence of water soluble phenolics. Test for TerpenoidsSalkowski test:
2 mL of extract was mixed with 2 mL of chloroform in a test tube and 3 mL conc. H2SO4 was added along the sides of the test tube. The formation of reddish-brown colour is indicative of the presence of terpenoids.Test for monoterpenes:
A few drops of 10% vanillin in ethanol were added to 2 mL of extract and mixed well. Then few drops of conc. H2SO4 were added. Formation of reddish-brown colour is indicative of the presence of terpenoids.
Sesquiterpenes test:
1 mL conc. H2SO4 was added to 2 mL of extract and mixed well. A brown, green, red or blue colour indicated the presence of terpenoids.
Test for SteroidsLiberman Burchard Test:
2 mL acetic anhydride and 2 mL of conc. H2SO4 were added to 2 mL of extract and mixed well. The formation of a blue or green colour indicated the presence of steroids.Test for Cardiac glycosides:
One millilitre of glacial acetic acid was added to 3 mL of extract, and conc. H2SO4 was introduced to the bottom of the tube. A reddish-brown or violet-brown ring at the interface of the two liquids indicated the presence of cardiac glycosides.
Determination of Heavy Metals
The Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (iCAP Q, Thermo Fisher Scientific, Breman, Germany) was used to detect the heavy metals (Pb, Cd, As, Hg) in the dry powder of P. niruri Linn. whole plant according to microwave digested ICPMS method.
Homogenized sample was weighed accurately to four decimal places, into a microwave digester reactor tube. Conc. HNO3 (10 mL) was added to theTeflon microwave digester tube. Only conc. HNO3 10 mL) was added to digester tubes as blank. The next four tubes were also placed in the microwave digester (Model: Varian) along with the samples, and digestion was done as per the program. After 15 minutes, the reactors containing the samples were removed and allowed to cool. The reactors were then opened, and pressure was carefully released by opening the valves.
The samples were transferred to a volumetric flask (50 mL) by rinsing Teflon tubes three times with de-ionized water and making up the volume to the 50 mL mark and calculation of heavy metal concentration was done. as follows:
Development of Thin Layer Chromatography (TLC) and High-Performance Thin Layer Chromatography (HPTLC) fingerprints
Preparation of extracts for TLC and HPTLC studies:
200 g of powdered plant material was refluxed with 750 mL of methanol for six hours and then filtered. The extract was dried by evaporation using a rotary evaporator (Buchi, Rotavapor R-210, Switzerland) at 45 °C, and the resulting methanol extract was stored in a refrigerator for further investigation.
Chromatographic materials:
1 g of methanol extract was dissolved in 5 mL of methanol. The extract was applied onto silica gel G with fluorescent (Al foils) Thin Layer Chromatography (TLC) plates (Sigma Aldrich: 20 × 20 cm; with thickness 0.2 – 0.3 mm), which were activated at 100 °C for 30 minutes and brought to room temperature just beforeuse. The methanol extract (10 μL) was applied 1 cm above the edge of the chromatographic plates and developed in an airtight chamber previously saturated with 30 mL of the solvent system.
Development of TLC and HPTLC:
Thin-layer chromatography was performed to develop a chromatographic profile of the plant extract using an optimised solvent system. The samples were applied onto TLC plates and developed in a mobile phase consisting of Cyclohexane: Methanol: Ethyl acetate in the ratio of 3.5:0.50:0.25. The developed chromatogram was visualised under UV light at 254 nm and 366 nm to detect characteristic bands. Subsequently, the optimised TLC method was subjected to HPTLC analysis using CAMAG HPTLC system (CAMAG, Switzerland) equipped with a Linomat 5 applicator and a CAMAG TLC scanner 3, operated using Wincats software (Version 1.44).
RESULTSPharmacognostical characteristics of P. niruri Linn:
The macroscopic and microscopic features of P. niruri Linn. were recorded as follows.Macroscopic features
The root is small, 2.50 – 9.50 cm long, nearly straight, gradually tapering, and bears several fibrous secondary and tertiary roots. The external surface of the root is light brown. The stems are slender, glabrous, cylindrical, and greenish brown in colour. Each stem bears five to ten pairs of leaves, and the internodes are 1.00 – 3.50 cm long. The leaves are compound, with leaflets arranged in two rows along the rachis; they are alternate, opposite, and decussate, and are almost sessile (Figure 1). The leaflets are up to 1.50 cm long and 0.50 cm wide, greenish-brown in colour, and slightly bitter in taste.

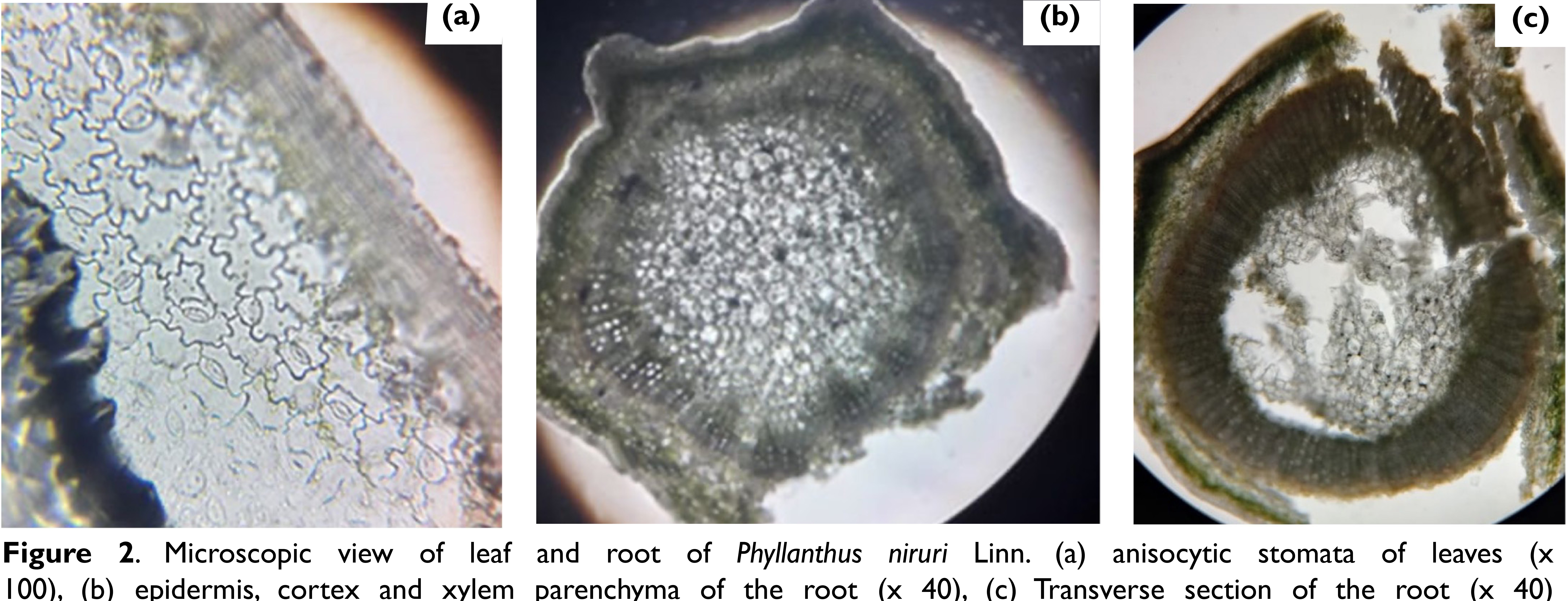
Microscopic features
The transverse section of the root shows 4–6 layers of cork, consisting of thin-walled, rectangular cells that are tangentially elongated and radially arranged. The secondary cortex consists of 8–10 layers of thin walled, tangentially elongated parenchymatous cells. The secondary phloem is narrow and composed of sieve elements.
The transverse section of the stem shows a single-layered epidermis, composed of thick-walled, flattened, tangentially elongated cells. The cortex comprises 4–6 layers of oval, tangentially elongated, thinwalled parenchymatous cells. Some cortical cells contain yellowish-brown contents. Endodermis is distinct, and the pericycle is represented by a discontinuous ring, composed of several tangentially elongated strands of lignified fibres with thick walls and narrow lumens.
The transverse section of the leaf shows a biconvex outline, single-layered epidermis on both sides, covered externally by a thick cuticle, and a palisade layer present beneath the upper epidermis. The epidermis on either side is composed of thin-walled, tangentially elongated cells, externally covered by a thick cuticle, anisocytic-type stomata present on both epidermises (Figure 2).
Physico-chemical characteristics
Foreign matter, moisture content, total ash, water-soluble ash, acid-insoluble ash, hot- and cold-water extractive values, and hot- and cold-methanol extractive values of P. ninuri are shown in Table 1.
| Table 1. Physico-chemical parameters of Phyllanthus niruri Linn. | |
|
Physico-chemical properties |
Parameter (%) (mean, standard deviation) |
|
Foreign matter % w/w |
1.89 |
|
Moisture content % w/w |
11.40 ±0.08 |
|
Total ash content % w/w |
5.26 ±0.10 |
|
Water-soluble ash content % w/w |
0.05 ±0.00 |
|
Acid-insoluble ash content % w/w |
5.25 ±0.04 |
|
Extractable matters |
|
|
Solvent: Water |
|
|
Cold % w/w |
8.90 ±0.10 |
|
Hot % w/w |
23.02 ±0.80 |
| Solvent: Methanol | |
|
Cold % w/w |
7.20 ±1.50 |
|
Hot % w/w |
10.40 ±0.80 |
Qualitative phytochemical analysis
Qualitative phytochemical analysis indicated the presence of major secondary metabolites, including saponins, tannins, flavonoids, phenols, terpenoids, steroids, and cardiac glycosides, in both hot-water and hot-methanol extracts of P. niruri whole plant, as shown in Table 2.
| Table 2. Qualitative phytochemical parameters of Phyllanthus niruri Linn. whole plant extract | |||
| Phyto constituent | Test | Hot-water extract | Hot-methanol extract |
|---|---|---|---|
| Saponins | Frothing test | + | - |
| Tannins | FeCl3 test |
+++ (Blue-black precipitate) |
+++ (Blue-black precipitate) |
| Pb(OAc)2 test |
+++ (Yellow precipitate) |
+++ (Yellow precipitate) |
|
| Vanillin test | - | - | |
| Alkaloids | Mayer’s reagent | - | - |
| Picric acid test | - | - | |
| Tannic acid test | - | - | |
| Wagner test | +
(Dark red) |
- | |
| Flavonoids | Ammonia test | - | - |
| Aluminium Chloride test | - | - | |
| Shinoda test |
+++ (Dark orange) |
++ (orange) |
|
| Phenols | Folin reagent | ++ | + |
| Pb(OAc)₂ test |
+++ (Yellow precipitate) |
+++ (Yellow precipitate) |
|
| Vanillin test | - | - | |
| FeCl₃ test | +++ (Blue-black precipitate) |
+++ (Blue-black precipitate) |
|
| Terpenoids | Salkowski test |
+ (Reddish-brown colour) |
+++ (Dark reddish-brown colour) |
| Test for monoterpenes |
++ (Red colour) |
+++ (Dark red colour) |
|
| Sesquiterpenes test |
++ (Red colour) |
++ (Brown-green colour) |
|
| Steroids | Liberman Burchard Test | ++ (Dark bluish-green colour) | ++ (Bluish-green colour) |
| Cardiac glycosides | +++ Reddish-brown ring formed |
+++ Reddish-brown ring formed |
|
| + test positive – test negative |
|||
Determination of heavy metals
The levels of Pb, Cd, As, and Hg in the P. niruri Linn. whole plant were below detectable limits (<0.05 mg/kg) as shown in Table 3.
| Table 3. Assessment of heavy metal in Phyllanthus niruri Linn. whole plant | |
|
Heavy metal |
Concentration (mg/kg) |
|
Pb |
Not detected (<0.05) |
|
Cd |
Not detected (<0.05) (<0.05) |
|
As |
Not detected (<0.05) |
|
Hg |
Not detected (<0.05)(<0.05) |
|
*Limit of detection = 0.05 mg/kg |
|
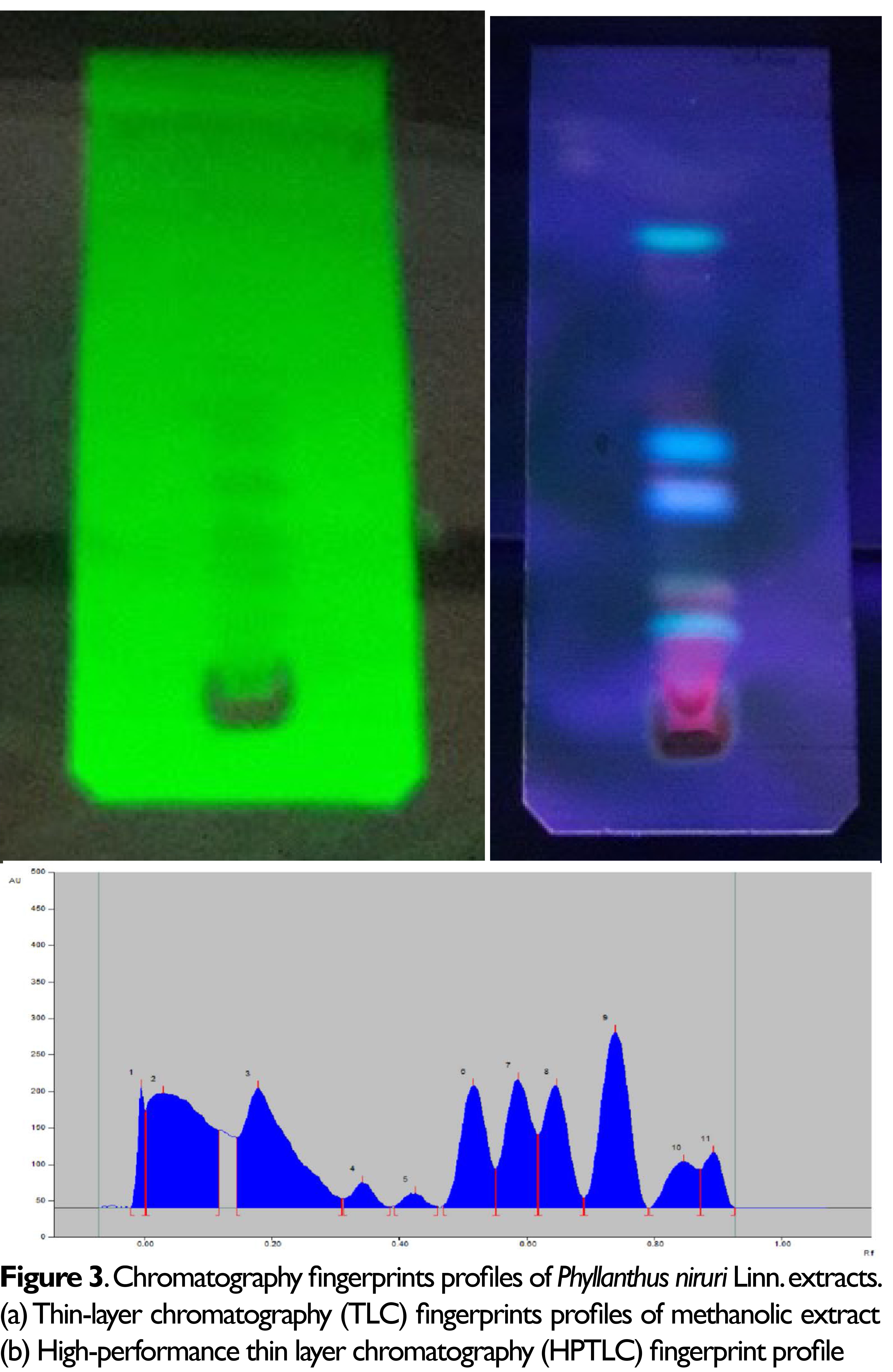
TLC and HPTLC studies
TLC profile was observed in P. niruri bearing prominent Rf values of 0.30, 0.34 and 0.56 at 254 nm. Additional prominent spots were observed in P. niruri bearing Rf value of 0.20, 0.40, 0.50 and 0.80 (at 366 nm) and HPTLC profile showed 10 peaks related to TLC (Figure 3).
DISCUSSION
In this study, the macroscopic and microscopic identification of P. niruri Linn. was performed for the whole plant, and the results were compared with the references provided by the Ayurvedic Pharmacopoeia of India, a collection of monographs systematically done for Ayurvedic medicinal plants [11]. The experimental results were comparable with the descriptions in the Ayurvedic Pharmacopoeia of India. The results of this study describes the baseline macroscopic and microscopic standards P. niruri.
Ash values of herbal raw materials serve as critical indicators of the quality and purity of crude plant materials, helping to identify the presence of various impurities such as carbonates, oxalates, and silicates. Analysis of water-soluble ash content allows for the quantification of inorganic compounds, whereas examination of acid-insoluble ash content can reveal the presence of silica and contamination with earthy materials. The ash values of the plant material of this study were measured according to the standard methods, and the results were comparable with the reference values reported in the Ayurvedic Pharmacopoeia of India [11]. Similar studies have reported total ash content of the whole P. niruri plant ranging from 6.23 ± 0.41% in India to 7.7 ± 0.2% in Sri Lanka, which is comparable to the findings of the present study [14,15]. Water-soluble ash content was similar to that reported in the Indian study (0.23 ± 0.08) [15]. However, the acid-insoluble ash content observed in the present study was higher than that reported in both the Indian study and the previous study done in Sri Lanka. This variation may be attributed to differences in geographical location and soil conditions.
The estimation of extractable matter determines the quantity of active constituents present in a specific amount of plant material extracted using a selected solvent. Extraction of any plant material using a particular solvent yields a mixture containing various phytochemicals. The composition of these phytochemicals depends on the nature of the plant material and the solvent used. This analysis also provides an indication of whether the crude drug is fully exhausted [16]. The extractable matters of P. niruri were comparable with the values mentioned in Ayurvedic Pharmacopoeia of India [11]. Research findings revealed that the cold-water extractable matter of P.niruri was 18.6% [17]. However, the present study showed a lower cold-water extractable matters value of 8.9%. The highest extractable matter observed in this study was the hot water extractable fraction (23%) indicating the presence of considerable amount of phytoconstituents in hot water extract.
Herbal materials may pose health risks due to the presence of toxic substances, such as Hg, As, Cd, and Pb [18]. As per the World Health Organization recommendations, herbal medicines should either be free of heavy metals or within permissible limits. These heavy metals can accumulate in various plant parts through the plant’s biological cycle [19]. The concentration of Hg, As, Cd, and Pb in the whole plant of P. niruri were below the detectable limits. The absence of these toxic heavy metals indicates that the plant material is free from significant metal contamination and meet essential safety criteria for medical or nutraceutical use. This also suggests that the environmental conditions of the collection site were not polluted with heavy metals.
Phytochemical analysis is essential for assessing the quality and therapeutic potential of herbal materials. Previous studies on P. niruri have demonstrated its richness in bioactive compounds, such as phenols, tannins, flavonoids, terpenoids, and cardiac glycosides[20]. These compounds are associated with significant therapeutic potential, particularly in the management of respiratory, urinary and liver disorders [6]. In the present study, qualitative analysis of hot water and hot methanol extracts of P. niruri confirmed the presence of phenols, flavonoids, tannins, terpenoids, and cardiac glycosides. These findings are comparable with the preliminary phytochemical investigations conducted in India and in Sri Lanka that reported the presence of saponins, tannins, phenols and flavonoids [14,17]. Furthermore, alkaloids were not detected in either study indicating a consistent absence of this compound class.
Chromatographic analysis methods are extensively used in pharmaceutical industries for accurate identification of raw materials and assessment of herbal material quality [21]. HPTLC is an advanced, modern adaptation of TLC, offering enhanced separation efficiency and improved detection limits. The HPTLC fingerprint patterns of the methanolic extract of P. niruri revealed the presence of multiple phytoconstituents. The Rf values at 254 nm correspond to three UV-absorbing compounds, likely phenolic or aromatic in nature. Other additional compounds visible under long-wave UV light were possibly flavonoids or other fluorescent phytochemicals. Previous research done in Sri Lanka also showed the comparable results related to TLC fingerprint pattern and densitogram profiles [14].
LimitationsThe present study is limited to using plant material collected from a single geographical location and season, which may not account for possible variations in pharmacognostical and phytochemical parameters. Phytochemical analysis was limited to qualitative screening and TLC-HPTLC fingerprinting, without quantitative estimation or structural characterization of individual bioactive compounds. Further studies addressing these aspects would strengthen the standardization of P. niruri Linn.
CONCLUSIONThis study describes the pharmacognostical, physicochemical and phytochemical characteristics of Phyllanthus niruri Linn. Microscopic and organoleptic evaluations established the identity of the plant material. Physicochemical parameters such as moisture content, ash values and extractive values fell within acceptable limits, indicating good quality and purity. Qualitative phytochemical screening revealed the presence of major bioactive constituents, including saponins, tannins, flavonoids, phenols, terpenoids, steroids and cardiac glycosides, in both aqueous and methanolic extracts. Heavy metals such as Pb, Cd, As and Hg were below detectable levels.
Declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Funding
This study was funded by the University Grants Commission, University of Colombo, Sri Lanka (UGC/VCDRIC/PG2016(II)/IIM/02, 02.02.2017). The funder had no role in the design and conduct of this study.
Availability of data materials
All data generated or analysed during this study are available from the corresponding author upon request.
Declaration of Artificial Intelligence Use
The authors confirm that this manuscript was prepared, analysed, and written solely by human effort, without the assistance of generative AI or AI-based technologies.
Author contributions
Conceptualization: JMD, PKP
Software: PKP
Investigation, Visualization, Writing – original draft: JMD
Supervision: PKP, PG, LDAMA
Validation, Resources, Data curation, Project administration, Funding acquisition, Writing – review & editing: JMD, PKP, PG, LDAMA
Received: 20 September, 2025 Accepted:22 January, 2026 Published online: 09 February, 2026References
- Hoffmann P, Kathriarachchi H, Wurdack KJ. A phylogenetic classification of Phyllanthaceae (Malpighiales; Euphorbiaceae sensu lato). KewBull. 2006;61: 37–53. Available: https://www.researchgate.net/publication/ 286111396_A_phylogenetic_classification_of_Phyllanthaceae_Malpighiales_Euphorbiaceae_sensu_lato
- Iizuka T, Nagai M, Taniguchi A, Moriyama H, Hoshi K. Inhibitory Effects of Methyl Brevifolincarboxylate Isolated from Phyllanthus niruri L. on Platelet Aggregation. Biol Pharm Bull. 2007;30: 382–384. doi:10.1248/ bpb.30.382
- Botany Brisbane. Phyllanthacea. [cited 24 Jan 2026]. Available: https:// www.botanybrisbane.com/plants/phyllanthaceae/
- Ramalho SD, Pinto MEF, Ferreira D, Bolzani VS. Biologically Active Orbitides from the Euphorbiaceae Family. Planta Med. 2018;84: 558–567. doi:10.1055/S-0043-122604
- Jayaweera DMA, Senaratna LK. Medicinal Plants (Indigenous and Exotic) Used in Ceylon PART II. 1st ed. Colombo: The National Science Foundation, Sri Lanka; 2006. Available: https://www.siddha.jfn.ac.lk/ wp-content/uploads/2020/08/materiamedica2-Jeyaweera.pdf
- Basavaraju M, Gunashree B.S. Phyllanthus Niruri L: A Holistic Medicinal Plant with Modern Therapeutics. 1st ed. Medicinal Plants. Scientist R Academy; 2022. Available: https://www.scientistracademy.com/ about-8
- Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. Journal of Pharmacy and Pharmacology. 2006;58: 1559–1570.doi:10.1211/JPP.58.12.0001
- Lee NYS, Khoo WKS, Adnan MA, Mahalingam TP, Fernandez AR, Jeevaratnam K. The pharmacological potential of Phyllanthus niruri. Journal of Pharmacy and Pharmacology. 2016;68: 953–969. doi:10.1111/ JPHP.12565
- Shetty RG, Harsha R. Adulteration in medicinal plants and herbal drugs. International Journal on Agricultural Sciences. 2021;12: 12–17. doi:10.53390/ijas.v12i1.4
- Chanda S. Importance of pharmacognostic study of medicinal plants: An overview. J Pharmacogn Phytochem. 2014;2: 69–73. Available: https://www.phytojournal.com/archives/2014/vol2issue5/Part- B/28.1.pdf.
- Government of India. The Ayurvedic Pharmacopoeia of India. Government of India; 1986. Available: https://www.ayurveda.hu/api/APIVol- 1.pdf.
- Goveas SW, Abraham A. Extraction and secondary metabolite analysis of Coscinium fenestratum (Gaertn.) Colebr: an important medicinal plant of Western Ghats. Int J Pharm Sci Res. 2014;5: 3484–3489. doi:http://dx.doi.org/10.13040/IJPSR.0975-8232.5(8).3484-89.
- Dahanayake JM, Perera PK, Galappatty P, Perera HDSM, Arawwawala LDAM. Comparative Phytochemical Analysis and Antioxidant Activities of Tamalakyadi Decoction with Its Modified Dosage Forms. Evidence-based Complementary and Alternative Medicine. 2019;2019: 6037137. doi:10.1155/2019/6037137.
- Perera HARP, Karunagoda K, Perera PK, Samarasingha K, Arawwawala LDAM. Phyllanthus niruri Linn grown in Sri Lanka: Evaluation on phyto and physico-chemical properties of the whole plant. World J Pharm Pharm Sci. 2015;4: 1452–1459. Available: https://www.researchgate. net/publication/283718998_Phyllanthus_niruri_Linn_grown_in_Sri_Lanka_Evaluation_on_phyto_and_physico-chemical_properties_of_the_whole_plant.
- Khatoon S, Rai V, Rawat AKS, Mehrotra S. Comparative pharmacognostic studies of three Phyllanthus species. J Ethnopharmacol. 2006;104: 79–86. doi:10.1016/J.JEP.2005.08.048.
- Samali A, Florence DT, Odeniran OA, Cordelia ON. Evaluation of chemical constituents of Phyllanthus Niruri. Afr J Pharm Pharmacol. 2012;6: 125–128.doi:10.5897/AJPP10.363
- Bhasme SN, Khan N. Pharmacognostic and phytochemical perspectives of Phyllanthus niruri. Neuro Quantology. 2022;20: 6659–6668. doi:10.14754/nq.2022.20.9.NQ44780
- Luo L, Wang B, Jiang J, Fitzgerald M, Huang Q, Yu Z, et al. Heavy Metal Contaminations in Herbal Medicines: Determination, Comprehensive Risk Assessments, and Solutions. Front Pharmacol. 2021;11. doi:10.3389/fphar.2020.595335
- Vinogradova N, Glukhov A, Chaplygin V, Kumar P, Mandzhieva S, Minkina T, et al. The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae. 2023;9. doi:10.3390/horticulturae9020239
- Linda PS, Pyare K. Qualitative phytochemical analysis of Acalpha Indica L. medicinal plants of family Euphorbiaceae. Modern Management, Applied Science & Social Science (IJEMMASSS). 2024;06: 258–261.doi:10.62823/6.2(II).6730
- Karthika K, Paulsamy S. TLC and HPTLC Fingerprints of Various Secondary Metabolites in the Stem of the Traditional Medicinal Climber, Solena amplexicaulis. Indian J Pharm Sci. 2015;77: 111. doi:10.4103/0250- 474X.151591
